Metal corrosion is the spontaneous destruction of metal as a result of chemical or physico-chemical interaction with the environment.
Zinc and its anti-corrosion properties
Properties of zinc, which determine the effectiveness of its use for anti-corrosion protection of steel. Zinc is a silvery-white, rather brittle metal under normal conditions with a density of ~7.1 g/cm3 and a melting point of about 420°C.
Just like iron, zinc belongs to the group of metals with increased thermodynamic instability, having an electrode potential value less than the potential of a hydrogen electrode at pH = 7 (-0.413 V).
However, water has almost no effect on zinc. This is explained by the fact that when zinc interacts with water, a hydroxide is formed on its surface, which is practically insoluble and prevents the further course of the reaction. Even in a slightly acidic environment, the corrosion of pure zinc is slowed down, which is associated with a fairly high value of the overvoltage of hydrogen evolution on zinc (~1 V).
In air, zinc oxidizes, becoming covered with a thin but durable film of zinc oxide or basic zinc carbonate. This film reliably protects it from further oxidation and provides high corrosion resistance.
In contrast, rust, for example, does not form a continuous film on the surface of iron and between individual crystals of hydrated ferric oxide, there are large gaps, the presence of which explains the tendency of iron to progressive corrosion.
The high anti-corrosion properties of zinc when applied to iron (steel) are also due to the fact that zinc has an electrochemical potential lower than iron (-760 and -440 mV, respectively), therefore, in the electrochemical zinc-iron pair that occurs in the presence of water ( moisture), zinc acts as an anode and dissolves, and the metal substrate (iron) acts as a cathode:
Zn – 2e ↔ Zn2 + H2O + ½O2 + 2e ↔ 2OH¯
As a result, steel passivation occurs due to alkalization.
Zinc ions react with carbon dioxide in the air. This is accompanied by the formation of dense layers of insoluble zinc carbonates, which inhibit the further development of the corrosion process.
Three mechanisms of anti-corrosion protection of metal
- Barrier - creating an impenetrable or low-permeable coating that prevents the penetration of external aggressive environment(moisture, oxygen and other oxidizing agents);
- Inhibiting - increasing the corrosion resistance of the metal and slowing down the corrosion rate due to chemical and electrochemical processes with the participation of special components introduced into the coating composition (corrosion inhibitors);
- Cathodic - electrochemical protection of metal structures with protective zinc-filled primers, based on the difference between the standard electrode potentials of steel and zinc, which acts as a sacrificial anode.
Metal tread protection
Sacrificial protection of metal is a method of anti-corrosion protection in which the protected surface must be provided with contact with a more active metal. In relation to iron, the more active metals are cadmium, chromium, zinc, magnesium and other metals.
From the mechanism of metal corrosion, it follows that more active metal begins to emit electrons and attach to the formed ions of the hydroxyl group from the electrolyte solution, and the other, less active, will accept electrons, attaching them to its ions. As a result, the more active metal - the anode - will be oxidized, and the less active metal - the cathode - will be reduced. Thus, the anode will protect against corrosion
As a result, anode will oxidize and cathode restore
Protective protection has found wide application for the protection of such objects as: underground pipelines, tanks, sea and river vessels, etc. All these objects are in constant contact with the electrolyte, whether groundwater, chemical solutions, sea or river water.
To implement tread protection, it is necessary to ensure contact of the protector itself with the clean surface of the protected metal (see figure)

If this structure is exposed to the external environment, then the electrons of the protector will pass into the protected metal and the evolution of hydrogen will begin at the cathode. Protector ions, combining with oxygen (hydroxyl groups OH), cause oxidative reaction, which leads to the appearance of hydroxide of the metal from which the protector is made. This ensures cathodic protection of the metal until the protector is completely destroyed due to corrosion. After complete destruction, the metal itself will begin to corrode.
Cold galvanizing
Among paint and varnish materials, a class of protective primers (zinc-filled or zinc-containing) is distinguished. The use of this type of material is called cold galvanizing.
Origin of the term "Cold galvanizing"
The article “Reliable Russian coatings for industrial facilities” by the VMP company, Yekaterinburg (Magazine “Industrial Painting” No. 05-06), contains the following information:
“The ZINOL coating containing 96% (wt.) zinc has a measured potential close to the values characteristic of hot-dip galvanizing. It changes little over time and contributes to effective cathodic protection. When this fact was first established, the ZINOL coating, by analogy with hot galvanizing (i.e., a coating obtained by immersion in molten zinc), was called “cold”; the term took root in the market and was later extended to other zinc-rich coatings applied by paint and varnish methods.”
Operation of protective zinc-rich primers
It is known that as zinc oxidizes, dense products form in micropores and on the coating surface chemical reaction, tread protection gradually decreases, and barrier protection increases. The relationship between the two protection mechanisms and the nature of their changes over time is individual for each material. Initially, the protective properties of the coating strongly depend on the content of zinc powder, its size, the nature of the packaging, and the nature of the film-forming substance. The higher the zinc content and the higher the electrical conductivity of the coating, the better they are expressed. But the less pronounced protective properties of zinc-filled coatings can be compensated by more pronounced barrier protection mechanisms.
Among all existing species The most common type of metal destruction is electrochemical corrosion, which occurs as a result of its interaction with an electrolytically conductive medium. The main reason for this phenomenon is the thermodynamic instability of metals in the environments that surround them.

Many objects and structures are susceptible to this type of corrosion:
- gas and water pipelines;
- elements of vehicles;
- other structures made of metal.
Corrosive processes, that is, rust, can occur in the atmosphere, in the soil, and even in salt water. Cleaning metal structures from deposits electrochemical corrosion is a complex and lengthy process, so it is easier to prevent its occurrence.
Main varieties
During corrosion in electrolytes, chemical energy is converted into electrical energy. In this regard, it is called electrochemical. It is customary to distinguish the following types of electrochemical corrosion.
Intercrystalline
Intergranular corrosion refers to a dangerous phenomenon in which the grain boundaries of nickel, aluminum and other metals are destroyed in a selective manner. As a result, the strength and plastic properties of the material are lost. The main danger of this type of corrosion is that it is not always visually noticeable.

Pitting
Pitting electrochemical corrosion is a point lesion of individual areas of the surface of copper and other metals. Depending on the nature of the lesion, closed, open, and superficial pitting are distinguished. The size of the affected areas can vary from 0.1 mm to 1.5 mm.

Slotted
Crevice electrochemical corrosion is commonly called the intensified process of destruction of metal structures in the locations of cracks, gaps and cracks. Crevice corrosion can occur in an air atmosphere, gas mixtures, as well as sea water. This type of destruction is typical for gas pipelines, the bottoms of sea vessels and many other objects.
Corrosion occurs in conditions of a small amount of oxidizer due to the difficult approach to the crack walls. This leads to the accumulation of corrosive products inside the gaps. The electrolyte contained in the internal space of the gap can change under the influence of hydrolysis of corrosion products.

In order to protect metals from crevice corrosion, it is common to use several methods:
- sealing gaps and cracks;
- electrochemical protection;
- inhibition process.
As preventive methods, you should use only those materials that are least susceptible to rust, and also initially correctly and rationally design gas pipelines and other important objects.
Competent prevention in many cases is a simpler process than subsequent cleaning of metal structures from ingrained rust.
How different types of corrosion manifest themselves
An example of the corrosion process is the destruction of various devices, car components, as well as any structures made of metal and located:
- in atmospheric air;
- in waters - seas, rivers contained in the soil and under layers of soil;
- in technical environments, etc.

During the rusting process, the metal becomes a multi-electron galvanic cell. So, for example, if copper and iron come into contact in an electrolytic medium, copper is the cathode and iron is the anode. By donating electrons to copper, iron in the form of ions enters the solution. Hydrogen ions begin to move towards copper and are discharged there. Becoming more and more negative, the cathode soon becomes equal to the potential of the anode, as a result of which the corrosion process begins to slow down.
Different types of corrosion manifest themselves in different ways. Electrochemical corrosion manifests itself more intensely in cases where the cathode contains inclusions of metal with less activity compared to the corroding one - rust appears on them faster and is quite expressive.
Atmospheric corrosion occurs in humid air and normal temperatures. In this case, a film of moisture with dissolved oxygen forms on the surface of the metal. The process of metal destruction becomes more intense as air humidity and the content of gaseous oxides of carbon and sulfur increase, provided that:
- cracks;
- roughness;
- other factors that facilitate the condensation process.

Soil corrosion most affects a variety of underground structures, gas pipelines, cables and other structures. The destruction of copper and other metals occurs due to their close contact with soil moisture, which also contains dissolved oxygen. Destruction of pipelines can occur as early as six months after their construction if the soil in which they are installed is characterized by high acidity.
Under the influence of stray currents emanating from foreign objects, electrical corrosion occurs. Its main sources are electrical railways, power lines, as well as special installations operating on direct current. To a greater extent, this type of corrosion provokes destruction:
- gas pipelines;
- all kinds of structures (bridges, hangars);
- electrical cables;
- oil pipelines.
The action of the current provokes the appearance of electron entry and exit areas - that is, cathodes and anodes. The most intense destructive process is in areas with anodes, so rust is more noticeable there.
Corrosion of individual components of gas and water pipelines can be caused by the fact that the installation process is mixed, that is, it occurs using different materials. The most common examples are pitting corrosion that occurs in copper elements, as well as corrosion of bimetals.
With a mixed installation of iron elements with copper and zinc alloys, the corrosion process is less critical than with copper casting, that is, with alloys of copper, zinc and tin. Pipeline corrosion can be prevented using special methods.

Methods of rust protection
Various methods are used to combat insidious rust. Let's look at those that are the most effective.
Method No. 1
One of the most popular methods is electrochemical protection of cast iron, steel, titanium, copper and other metals. What is it based on?
Electrochemical processing of metals is a special method aimed at changing the shape, size and roughness of the surface by anodic dissolution in an electrolyte under the influence of electric current.
To ensure reliable protection against rust, it is necessary to treat metal products with special means, which contain various components of organic and inorganic origin, even before using them. This method allows you to prevent rust from appearing for a certain time, but later you will have to renew the coating.
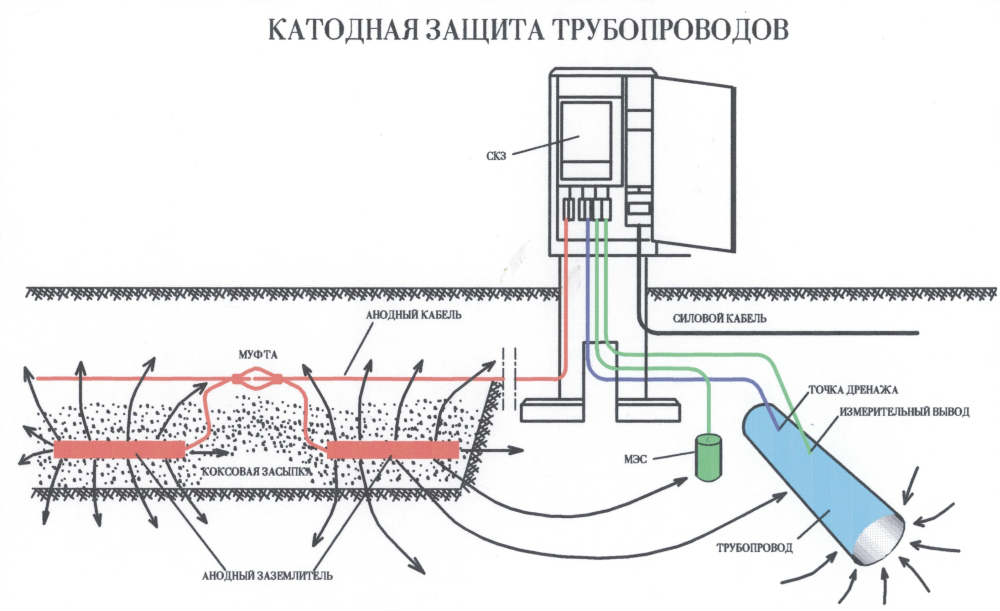
Electrical protection is a process in which a metal structure is connected to an external source of constant electric current. As a result of this, polarization of cathode-type electrodes is formed on its surface, and all anode regions begin to transform into cathode ones.
Electrochemical processing of metals can occur with the participation of an anode or cathode. In some cases, alternating processing of a metal product with both electrodes occurs.
Cathodic corrosion protection is necessary in situations where the metal to be protected does not have a predisposition to passivation. An external current source is connected to the metal product - a special cathodic protection station. This method is suitable for protecting gas pipelines, as well as water supply and heating pipelines. However, this method has certain disadvantages in the form of cracking and destruction of protective coatings - this occurs in cases of a significant shift in the potential of the object in the negative direction.
Method No. 2
Electric spark processing of metals can be carried out using installations various types– non-contact, contact, and also anode-mechanical.
Method No. 3
To reliably protect gas pipelines and other pipelines from rust, a method such as electric arc spraying is often used. The advantages of this method are obvious:

- significant thickness of the protective layer;
- high level performance and reliability;
- use of relatively inexpensive equipment;
- simple technological process;
- possibility of using automated lines;
- low energy costs.
Among the disadvantages of this method are low efficiency when processing structures in corrosive environments, as well as insufficient adhesion strength to the steel base in some cases. In any other situations, such electrical protection is very effective.
Method No. 4
To protect a variety of metal structures - gas pipelines, bridge structures, all kinds of pipelines - effective anti-corrosion treatment is required.

This procedure is carried out in several stages:
- thorough removal of fatty deposits and oils using effective solvents;
- cleaning the treated surface from salts soluble in water is carried out using professional high-pressure apparatus;
- removal of existing structural errors, alignment of edges - this is necessary to prevent chipping of the applied paint coating;
- thorough cleaning of the surface using a sandblaster - this is done not only to remove rust, but also to give the desired degree of roughness;
- application of anti-corrosion material and an additional protective layer.
Correct pre-treatment of gas pipelines and all kinds of metal structures will provide them with reliable protection from electrochemical corrosion during operation.
Electrochemical protection of metal structures from corrosion is based on the imposition of a negative potential on the protected product. It demonstrates a high level of efficiency in cases where metal structures are subject to active electrochemical destruction.
1 The essence of anti-corrosion electrochemical protection
Any metal structure begins to deteriorate over time as a result of corrosion. For this reason, before use, metal surfaces are necessarily coated with special compounds consisting of various inorganic and organic elements. Such materials reliably protect the metal from oxidation (rusting) for a certain period. But after some time they need to be updated (new compounds applied).
Then, when the protective layer cannot be renewed, corrosion protection of pipelines, car bodies and other structures is carried out using electrochemical techniques. It is indispensable for protecting against rusting tanks and containers operating underground, the bottoms of sea ships, various underground communications, when the corrosion potential (it is called free) is in the zone of repassivation of the base metal of the product or its active dissolution.
The essence electrochemical protection consists in the fact that a direct electric current is connected from the outside to a metal structure, which forms a cathode-type polarization of microgalvanic couple electrodes on the surface of the metal structure. As a result, the transformation of anodic regions into cathodic ones is observed on the metal surface. After such a transformation, the negative influence of the environment is perceived by the anode, and not the material itself from which the protected product is made.

Electrochemical protection can be either cathodic or anodic. With cathodic potential, the metal potential shifts to the negative side, and with anodic potential, it shifts to positive.
2 Cathodic electrical protection - how does it work?
The mechanism of the process, if you understand it, is quite simple. A metal immersed in an electrolytic solution is a system with a large number of electrons, which includes spatially separated cathode and anode zones, electrically closed to each other. This state of affairs is due to the heterogeneous electrochemical structure of metal products (for example, underground pipelines). Corrosion manifestations form on the anodic areas of the metal due to its ionization.

When attaching material with great potential(negative) to the base metal located in the electrolyte, the formation of a common cathode is observed due to the process of polarization of the cathode and anode zones. By high potential we mean a value that exceeds the potential of the anodic reaction. In the formed galvanic couple, a material with a low electrode potential dissolves, which leads to the cessation of corrosion (since the ions of the protected metal product cannot enter the solution).
The electric current required to protect the car body, underground tanks and pipelines, and the bottoms of ships can come from an external source, and not just from the functioning of a microgalvanic couple. In such a situation, the protected structure is connected to the “minus” of the electric current source. The anode, made of materials with a low degree of solubility, is connected to the “plus” of the system.
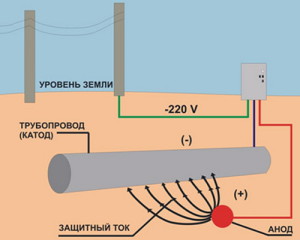
If the current is obtained only from galvanic couples, we speak of a process with sacrificial anodes. And when using current from an external source, we are talking about protecting pipelines, parts of vehicles and water vehicles with the help of superimposed current. The use of any of these schemes provides high-quality protection of the object from general corrosive decay and from a number of its special variants (selective, pitting, cracking, intergranular, contact).
3 How does the anodic technique work?
This electrochemical technique for protecting metals from corrosion is used for structures made of:
- carbon steels;
- passivating dissimilar materials;
- highly alloyed and;
- titanium alloys.
The anode scheme involves shifting the potential of the protected steel in a positive direction. Moreover, this process continues until the system enters a stable passive state. Such corrosion protection is possible in environments that are good conductors of electrical current. The advantage of the anodic technique is that it significantly slows down the rate of oxidation of the protected surfaces.

In addition, such protection can be carried out by saturating the corrosive environment with special oxidizing components (nitrates, dichromates and others). In this case, its mechanism is approximately identical to the traditional method of anodic polarization of metals. Oxidizers significantly increase the effect of the cathodic process on the steel surface, but they usually negatively affect the environment by releasing aggressive elements into it.
Anodic protection is used less frequently than cathodic protection, since many specific requirements are put forward for the protected object (for example, impeccable quality of welds of pipelines or a car body, constant presence of electrodes in the solution, etc.). In anode technology, cathodes are placed according to a strictly defined scheme, which takes into account all the features of the metal structure.

For the anodic technique, poorly soluble elements are used (cathodes are made from them) - platinum, nickel, stainless high-alloy alloys, lead, tantalum. The installation itself for such corrosion protection consists of the following components:
- protected structure;
- current source;
- cathode;
- special reference electrode.
It is allowed to use anodic protection for containers where mineral fertilizers, ammonia compounds, sulfuric acid are stored, for cylindrical installations and heat exchangers operated at chemical plants, for tanks in which chemical nickel plating is performed.
4 Features of tread protection for steel and metal
A fairly frequently used option for cathodic protection is the technology of using special protector materials. With this technique, an electronegative metal is connected to the structure. Over a given period of time, corrosion affects the protector, and not the protected object. After the tread wears down to a certain level, they put a new “defender” in his place.
Protective electrochemical protection is recommended for treating objects located in soil, air, water (that is, in chemically neutral environments). Moreover, it will be effective only when there is some transition resistance between the medium and the protector material (its value varies, but in any case it is small).

In practice, protectors are used when it is economically infeasible or physically impossible to supply the required charge of electric current to an object made of steel or metal. It is worth separately noting the fact that protective materials are characterized by a certain radius over which their positive effect extends. For this reason, you should correctly calculate the distance to remove them from the metal structure.
Popular protectors:
- Magnesium. They are used in environments with a pH of 9.5–10.5 units (soil, fresh and slightly salted water). They are made from magnesium-based alloys with additional alloying with aluminum (no more than 6–7%) and zinc (up to 5%). For the environment, such protectors that protect objects from corrosion are potentially unsafe due to the fact that they can cause cracking and hydrogen embrittlement of metal products.
- Zinc. These “protectors” are indispensable for structures operating in water with a high salt content. There is no point in using them in other environments, since hydroxides and oxides appear on their surface in the form of a thick film. Zinc-based protectors contain minor (up to 0.5%) additives of iron, lead, cadmium, aluminum and some other chemical elements.
- Aluminum. They are used in sea running water and at objects located on the coastal shelf. Aluminum protectors contain magnesium (about 5%) and zinc (about 8%), as well as very small amounts of thallium, cadmium, silicon, and indium.

In addition, iron protectors are sometimes used, which are made from iron without any additives or from ordinary carbon steels.
5 How is the cathode circuit performed?
Temperature changes and ultraviolet rays cause serious damage to all external components and components of vehicles. Protection of the car body and some of its other elements from corrosion by electrochemical methods is recognized as highly effective way extension of the ideal appearance cars.
The principle of operation of such protection is no different from the scheme described above. When protecting a car body from rusting, the function of an anode can be performed by almost any surface that is capable of efficiently conducting electric current (wet road surface, metal plates, steel structures). The cathode in this case is the vehicle body itself.

Elementary methods of electrochemical protection of a car body:
- We connect the body of the garage in which the car is parked through the mounting wire and an additional resistor to the battery positive. This protection against corrosion of the car body is especially effective in the summer, when the greenhouse effect is present in the garage. This effect precisely protects the external parts of the car from oxidation.
- We install a special grounding metalized rubber “tail” in the rear of the vehicle so that drops of moisture fall on it while driving in rainy weather. At high humidity, a potential difference is formed between the highway and the car body, which protects the outer parts of the vehicle from oxidation.
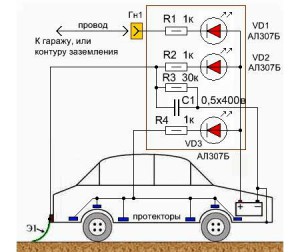
The car body is also protected using protectors. They are mounted on the thresholds of the car, on the bottom, under the wings. The protectors in this case are small plates made of platinum, magnetite, carboxyl, graphite (anodes that do not deteriorate over time), as well as aluminum and “stainless steel” (they should be replaced every few years).
6 Nuances of anti-corrosion protection of pipelines
Pipe systems are currently protected using drainage and cathodic electrochemical techniques. When protecting pipelines from corrosion using the cathodic scheme, the following are used:
- External current sources. Their plus will be connected to the anode grounding, and the minus to the pipe itself.
- Protective anodes using current from galvanic pairs.

The cathodic technique involves the polarization of the protected steel surface. In this case, underground pipelines are connected to the “minus” of the cathodic protection complex (in fact, it is a current source). “Plus” is connected to the additional external electrode using a special cable, which is made of conductive rubber or graphite. This scheme allows you to obtain a closed-type electrical circuit, which includes the following components:
- electrode (external);
- electrolyte located in the soil where the pipelines are laid;
- pipes directly;
- cable (cathode);
- current source;
- cable (anode).
For tread protection of pipelines, materials based on aluminum, magnesium and zinc are used, the efficiency of which is 90% when using protectors based on aluminum and zinc and 50% for protectors made of magnesium alloys and pure magnesium.

For drainage protection of pipe systems, technology is used to drain stray currents into the ground. There are four options for drainage piping - polarized, earthen, reinforced and straight. With direct and polarized drainage, jumpers are placed between the “minus” of stray currents and the pipe. For the earth protection circuit, it is necessary to make grounding using additional electrodes. And with increased drainage of pipe systems, a converter is added to the circuit, which is necessary to increase the magnitude of the drainage current.
Any metal products are easily destroyed under the influence of certain external factors, most often humidity. To prevent such phenomena, protective corrosion protection is used. Its task is to reduce the potential of the base material and thereby protect it from corrosion.
The essence of the procedure
Protective protection is based on a substance called an inhibitor. This is a metal with increased electronegative properties. When exposed to air, the protector dissolves. As a result, the base material is preserved even if it is severely affected by corrosion.
Various types of corrosion can be easily defeated if you use cathodic electrochemical methods, which include sacrificial protection. This procedure is an ideal solution when an enterprise does not have the financial capabilities or technological potential to provide complete protection against corrosion processes.
Main advantages
Protective protection of metals from corrosion is good way protection of any metal surfaces. Its use is advisable in several cases:
- When a company does not have enough production capacity to use more energy-intensive techniques.
- When you need to protect small structures.
- If protection of metal products and objects whose surfaces are coated with insulating materials is required.
To achieve maximum efficiency, it is advisable to use tread protection in an electrolytic environment.
When is protection required?
Corrosion occurs on any metal surfaces in a wide variety of areas - from the oil and gas industry to shipbuilding. Protective corrosion protection is widely used in painting tanker hulls. These vessels are constantly exposed to water, and special paint does not always prevent moisture reactions with the metal surface. The use of protectors is a simple and effective solution to the problem, especially if the vessels will be in operation for a long time.

Most metal structures are created from steel, so it is advisable to use protectors that have a negative electrode potential. Three metals are basic for the production of protectors - zinc, magnesium, aluminum. Due to the large potential difference between these metals and steel, the radius of protective action becomes wider, and any types of corrosion are easily eliminated.
What metals are used?

The protective system is built on the basis of various alloys, depending on the specific use of the protectors, for example, the environment in which it will be used. Protective corrosion protection is most often required for iron and steel products, but surfaces made of zinc, aluminum, cadmium or magnesium also require it. A special feature of sacrificial protection is the use of galvanic anodes, which protect pipes from soil corrosion. The calculation of such installations is carried out taking into account a number of parameters:
- current strength in the protector;
- indicators of its resistance;
- degree of protection required for 1 km of pipe;
- number of treads for the same segment;
- the distance that exists between the elements of the protective system.
Pros and cons of different protectors

Protection is built on the basis of protectors building structures from corrosion, pipelines different types(distribution, main, field). However, you need to use them wisely:
- the use of aluminum protectors is advisable in order to protect structures and structures in sea water and the coastal shelf;
- magnesium ones are suitable for use in weakly electrically conductive environments where aluminum and zinc protectors show low efficiency. But they cannot be used if it is necessary to protect the internal surfaces of tankers, tanks, and oil settling tanks, since magnesium protectors are characterized by an increased explosion and fire hazard. Ideally, projectors based on this element should be used for external protection of structures that are used in a fresh environment;
- zinc protectors are completely safe, so they can be used on any objects, even if they have a high level of fire hazard.
If the coating is paintwork

Very often it is necessary to protect an oil or gas pipeline from corrosion, taking into account the paint coating. Combining it with a protector is a passive way to protect structures from corrosion. At the same time, the effectiveness of such an event is not so high, but the following is achieved:
- defects on the coatings of metal structures and pipelines, for example, peeling, cracks, are leveled out;
- the consumption of protective materials is reduced, while the protection itself is more durable;
- the protective current is evenly distributed over the metal surface of the product or object.
Protective corrosion protection in combination with paint and varnish coatings is the ability to distribute protective current precisely to those surfaces that require maximum attention.
About pipeline protection

As you use it metal pipes are exposed to corrosion inside and outside. Plaque appears due to the fact that aggressive substances flow through the pipes, which react with the materials. The internal condition of metal products is affected by high levels of soil moisture. If high-quality protection of building structures from corrosion is not thought out, the following will happen:
- the pipeline will begin to collapse from the inside;
- it will be necessary to carry out preventive inspections of highways more often;
- more frequent repairs will be required, which will result in additional costs;
- it will be necessary to completely or partially stop an oil refining or other industrial complex.
There are several ways to protect pipelines - passive, active. Reducing the aggressiveness of the environment can also serve as a means of protection. To ensure comprehensive protection, the type of pipeline, the method of its installation and interaction with the environment are taken into account.
Passive and active methods of protection
All main methods of protecting pipelines from corrosion come down to performing a number of works. If we talk about passive methods, they are expressed as follows:
- a special installation method, when resistance to corrosion is thought out at the stage of pipeline installation. To do this, an air gap is left between the ground and the pipe, thanks to which neither groundwater, nor salts, nor alkalis will get inside the pipeline;
- applying special coatings to pipes that will protect the surface from soil influences;
- treatment with special chemicals, for example, phosphates, which form a protective film on the surface.
Protection scheme based active methods involves the use of electric current and electrochemical ion exchange reactions:

The case for tread protection
As you can see, there are many ways to improve the protective characteristics of pipelines and other metal products. But they all require the expenditure of electrical current. Protective protection against corrosion of pipelines is a more advantageous solution, since all oxide processes are stopped simply by applying alloys of other materials to the surface of metal pipes. The following factors speak in favor of this method:
- cost-effectiveness and simplicity of the process due to the absence of a direct current source and the use of magnesium, zinc or aluminum alloys;
- the possibility of using single or group installations, while the tread protection scheme is thought out taking into account the characteristics of the designed or already constructed facility;
- Possibility of use on any soil and in sea/ocean conditions where it is expensive or impossible to use external current sources.
Tread protection can be used to increase the corrosion resistance of various tanks, ship hulls, and tanks that are used in extreme conditions.
The following protection methods are distinguished.
1) Treatment of the external environment in which corrosion occurs. The essence of the method is either to remove from environment those substances that act as a depolarizer, or in isolating the metal from the depolarizer. For example, special substances or boiling are used to remove oxygen from water. Removing oxygen from a corrosive environment is called deaeration . The corrosion process can be slowed down as much as possible by introducing special substances into the environment - inhibitors . Volatile and vapor-phase inhibitors are widely used, which protect products made of ferrous and non-ferrous metals from atmospheric corrosion during storage, transportation, etc. The mechanism of action of inhibitors is that their molecules are adsorbed on the metal surface, preventing the occurrence of electrode processes.
A (-) Cr| H2O, O2 | Fe(+)K
at the anode: Cr - 2e→ Cr 2+
at the cathode: 2H 2 O+O 2 + 4e → 4OH -
Cr 2+ + 2 OH - → Cr(OH)2
Hydroxidechromium (II) is oxidized by atmospheric oxygen to Cr (OH) 3:
4 Cr(OH) 2 + 2H 2 O + O 2→ 4 Cr(OH) 3
Thus, as a result of electrochemical corrosion, the anodic coating is destroyed.
Cathode coatings . The cathode coating has a standard electrode potential more positive than that of the protected metal. As long as the coating layer isolates the metal from the environment, electrochemical corrosion does not occur. If the continuity of the cathode coating is damaged, it ceases to protect the metal from corrosion. Moreover, it even intensifies the corrosion of the base metal, because In the resulting galvanic couple, the anode is the base metal, which will be destroyed. An example is tin coating on iron (tinned iron). Let us consider the operation of the galvanic element that arises in this case.
A (-) Fe| H2O, O2 | Sn(+)K
at the anode: Fe - 2e→ Fe 2+
at the cathode: 2H 2 O+O 2 + 4e → 4OH -
Fe 2+ + 2 OH - → Fe(OH)2
The protected metal is destroyed. Thus, when comparing the properties of anodic and cathodic coatings, we can conclude that anodic coatings are the most effective. They protect the base metal even if the integrity of the coating is damaged, while cathodic coatings protect the metal only mechanically.
3) Electrochemical protection. There are two types of electrochemical protection: cathodic and sacrificial. In both cases, conditions are created for the appearance of a high electronegative potential on the protected metal.
Tread protection . The product being protected from corrosion is combined with scrap metal of a more electronegative metal (protector). This is equivalent to creating a galvanic cell in which the protector is the anode and will be destroyed. For example, to protect underground structures (pipelines), scrap metal (protector) is buried at some distance from them, attaching it to the structure (Figure 8.3).




