One of the most common and at the same time destructive factors affecting a car during operation is corrosion. Several methods have been developed to protect the body from it, and there are both measures aimed specifically against this phenomenon, as well as complex technologies for protecting the car, protecting it from various factors. This article discusses electrochemical protection of the body.
Causes of corrosion
Since the electrochemical method of protecting a car is aimed exclusively against corrosion, the reasons that cause it to damage the body should be considered. The main ones are water and road reagents used during the cold period. When combined with each other, they form a highly concentrated salt solution. In addition, dirt settled on the body retains moisture in the pores for a long time, and if it contains road reagents, it also attracts water molecules from the air.
The situation is aggravated if the car's paintwork has defects, even small ones. In this case, the spread of corrosion will occur very quickly, and even the remaining protective coatings in the form of primer and galvanization may not stop this process. Therefore, it is important not only to constantly clean the car from dirt, but also to monitor the condition of its paintwork. Temperature fluctuations as well as vibrations also play a role in the spread of corrosion.
You should also note the areas of the car that are most susceptible to corrosion. These include:
- parts located closest to the road surface, that is, sills, fenders and underbody;
- welds remaining after repairs, especially if they were carried out incorrectly. This is explained by high-temperature “weakening” of the metal;
- in addition, rust often affects various hidden, poorly ventilated cavities where moisture accumulates and does not dry out for a long time.

Operating principle of electrochemical protection
The considered method of protecting the body from rust is classified as active methods. The difference between them and passive methods is that the former create some kind of protective measures that do not allow corrosion-causing factors to affect the car, while the latter only isolate the body from exposure to atmospheric air. This technology was originally used to protect pipelines and metal structures from rust. The electrochemical method is considered one of the most effective.
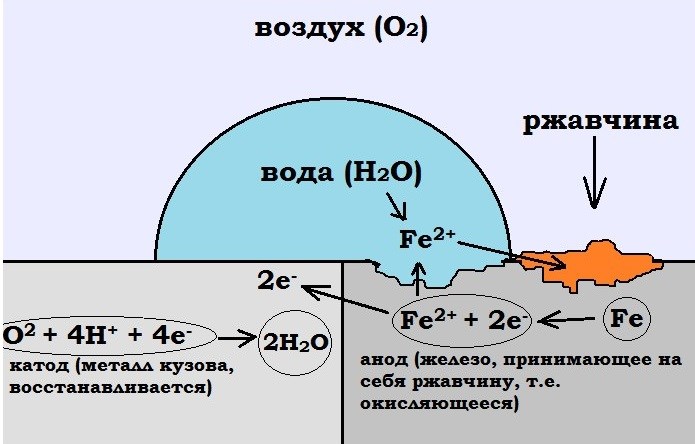
This method of body protection, which is also called cathodic, is based on the peculiarities of redox reactions. The essence is that a negative charge is applied to the protected surface.
The potential shift is carried out using an external direct current source or by connecting to a sacrificial anode consisting of a metal that is more electronegative than the object being protected.
The principle of operation of electrochemical protection of a car is that between the surface of the body and the surface of surrounding objects, due to the potential difference between them, a weak current passes through a circuit represented by moist air. Under such conditions, the more active metal undergoes oxidation, and the other, on the contrary, is reduced. This is why the protective plates made of electronegative metals used for cars are called sacrificial anodes. However, if the potential shifts excessively in the negative direction, hydrogen evolution, a change in the composition of the near-electrode layer, and other phenomena are possible, which lead to degradation of the protective coating and the occurrence of stress corrosion of the protected object.
The technology under consideration for cars involves using the body as a cathode (negatively charged pole), and various surrounding objects or elements installed on the car that conduct current, for example, metal structures or wet road surfaces, serve as anodes (positively charged poles). In this case, the anode must consist of an active metal, such as magnesium, zinc, chromium, aluminum.

Many sources give the potential difference between the cathode and anode. According to them, in order to create complete corrosion protection for iron and its alloys, it is necessary to achieve a potential of 0.1-0.2 V. Large values have little effect on the degree of protection. In this case, the protective current density should be from 10 to 30 mA/m².
However, these data are not entirely correct - in accordance with the laws of electrochemistry, the distance between the cathode and anode is directly proportional to the magnitude of the potential difference. Therefore, in each specific case it is necessary to achieve a certain value of the potential difference. In addition, air, considered as an electrolyte in this process, is capable of conducting electric current characterized by a large potential difference (approximately kW), so a current with a density of 10-30 mA/m² will not be conducted by air. Only a “side” current may occur as a result of the anode getting wet.
As for the potential difference, concentration polarization with respect to oxygen is observed. In this case, water molecules that fall on the surface of the electrodes are oriented towards them in such a way that electrons are released, that is, an oxidation reaction. At the cathode, this reaction, on the contrary, stops. Due to the absence electric current The release of electrons occurs slowly, so the process is safe and invisible. Due to the polarization effect, there is an additional shift in the body potential in the negative direction, which makes it possible to periodically turn off the corrosion protection device. It should be noted that the anode area is directly proportional to the effectiveness of electrochemical protection.
Creation options
In any case, the role of the cathode will be performed by the car body. The user needs to select an item that will be used as an anode. The choice is made based on the operating conditions of the vehicle:
- For cars that are stationary, a metal object located nearby, for example, a garage (provided that it is built of metal or has metal elements), or a ground loop, which can be installed in the absence of a garage in an open parking lot, can serve as a cathode.
- On a moving vehicle, devices such as a metallized rubber grounding “tail” and protectors (protective electrodes) mounted on the body can be used.
Due to the absence of current flowing between the electrodes, it is enough to connect the car’s on-board +12 volt network to one or more anodes through an additional resistor. Last device serves to limit the battery discharge current in the event of an anode-to-cathode short circuit. The main causes of short circuits are improper installation of equipment, damage to the anode or its chemical decomposition due to oxidation. Next, we discuss the features of using the previously listed items as anodes.
Using a garage as an anode is considered the most in a simple way electrochemical protection of the body of a stationary car. If the room has a metal floor or floor covering with exposed areas of iron reinforcement, then bottom protection will also be provided. During warm periods, a greenhouse effect is observed in metal garages, but if electrochemical protection is created, it does not destroy the car, but rather is aimed at protecting its body from corrosion.
Creating electrochemical protection in the presence of a metal garage is very simple. To do this, just connect this object to the positive connector battery car through an additional resistor and a mounting wire.
Even the cigarette lighter can be used as a positive connector, provided that there is voltage in it when the ignition switch is turned off (not all cars have this device that remains operational when the engine is turned off).

When creating electrochemical protection, the ground loop is used as an anode according to the same principle as the metal garage discussed above. The difference is that the garage protects the entire body of the car, while this method only protects its bottom. A grounding loop is created by driving four metal rods at least 1 m long into the ground around the perimeter of the car and stretching a wire between them. The circuit is connected to the car, as well as to the garage, through an additional resistor.
A rubber metallized grounding “tail” is the simplest way to electrochemically protect a moving vehicle from corrosion. This device is a rubber strip with metal elements. The principle of its operation is that in conditions of high humidity, a potential difference arises between the car body and the road surface. Moreover, the higher the humidity, the greater the effectiveness of the electrochemical protection created by the element in question. The grounding “tail” is installed in the rear of the car in such a way that it is exposed to splashes of water flying out from under the rear wheel when driving on a wet road surface, as this increases the efficiency of electrochemical protection.
The advantage of the grounding tail is that, in addition to the function of electrochemical protection, it relieves the car body from static voltage. This is especially true for vehicles transporting fuel, since an electrostatic spark, which is the result of the accumulation of a static charge during movement, is dangerous for the cargo it transports. Therefore, devices in the form of metal chains dragging along the road surface are found, for example, on fuel trucks.

In any case, it is necessary to isolate the grounding tail from the car body by direct current and, conversely, “short-circuit” it by alternating current. This is achieved by using an RC circuit, which is a basic frequency filter.
Protecting a car from corrosion by an electrochemical method using protective electrodes as anodes is also designed for operation while on the move. Protectors are installed in the most vulnerable areas of the body to corrosion, represented by sills, fenders, and bottom.
Protective electrodes, as in all previously discussed cases, operate on the principle of creating a potential difference. The advantage of the method under consideration is the constant presence of anodes, regardless of whether the car is stationary or moving. Therefore, this technology is considered very effective, but it is the most difficult to create. This is explained by the fact that to ensure high protection efficiency, it is necessary to install 15-20 protectors on the car body.
Elements made of materials such as aluminum, stainless steel, magnetite, platinum, carboxyl, and graphite can be used as protective electrodes. The first two options are classified as destructible, that is, the protective electrodes consisting of them need to be changed at intervals of 4-5 years, while the rest are called non-destructible, since they are characterized by significantly greater durability. In any case, the protectors are round or rectangular plates with an area of 4-10 cm².
In the process of creating such protection, you need to take into account some features of the protectors:
- the radius of protective action extends to 0.25-0.35 m;
- electrodes must be installed only on areas that have a paint coating;
- to secure the elements in question, epoxy glue or putty should be used;
- It is recommended to clean the gloss before installation;
- the outer side of the protectors must not be coated with paint, mastic, glue or other electrical insulating substances;
- Since the protective electrodes are positively charged capacitor plates, they must be insulated from the negatively charged surface of the car body.

The role of the dielectric gasket of the capacitor will be performed by the paint coating and glue located between the protectors and the car body. It should also be taken into account that the distance between the protectors is directly proportional to the electric field, so they should be installed at a small distance from each other to ensure sufficient capacitor capacity.
The wires are fed to the protective electrodes through punctures in the rubber plugs that cover the holes in the bottom of the car. You can install many small protectors or fewer protective electrodes on your car bigger size. In any case, it is necessary to use these elements in areas that are most vulnerable to corrosion, facing outward, since the role of the electrolyte in this case is performed by air.
After installing electrochemical protection of this type, the car body will not receive an electric shock, since it creates very little electricity. Even if a person touches the protective electrode, he will not receive a shock. This is explained by the fact that electrochemical anti-corrosion protection uses low-power direct current, creating a weak electric field. In addition, there is an alternative theory, according to which the magnetic field exists only between the surface of the body and the installation site of the protective electrodes. Therefore, the electromagnetic field created by electrochemical protection is more than 100 times weaker than the electromagnetic field of a mobile phone.
Sacrificial protection is a type of cathodic protection. A more electronegative metal - a protector - is attached to the protected structure, which, dissolving in the environment, protects the main structure from destruction. After complete dissolution of the protector or loss of contact with the protected structure, the protector must be replaced.
The protector works effectively if the transition resistance between it and environment not much. The tread action is limited to a certain distance. The maximum possible distance from the structure being protected is called the protective radius of the protector.
Sacrificial protection is used in cases where obtaining external energy for organizing cathodic protection is associated with difficulties, and the construction of special power lines is not economically profitable.
Protective protection is used to combat corrosion of metal structures in sea and river water, soil and other neutral environments. The use of protectors in acidic solutions is impractical due to the high rate of self-dissolution.
However, using pure metal as protectors is not always advisable. For example, pure zinc dissolves unevenly due to its coarse-grained dendritic structure, the surface of pure aluminum is covered with a dense oxide film, magnesium has a high rate of its own corrosion. To give the protectors the required performance properties, alloying elements are introduced into their composition.
(from hundredths to tenths of a percent), contributing to the required change in lattice parameters. Magnesium tread alloys contain A1 (5-7%) and Zn (2-5%) as alloying additives; content of impurities such as
Maintain at the level of tenths or hundredths of a percent. Iron as a tread material is used either in its pure form (Re-armco) or in the form of carbon steels.
or zinc oxide 2nO.
Aluminum protectors are used to protect structures operating in flowing sea water, as well as to protect port facilities and structures located on the coastal shelf.
Magnesium protectors are primarily used to protect small structures in weakly electrically conductive environments where the effectiveness of aluminum and zinc protectors is low - soils, fresh or slightly salty waters. However, due to the high rate of self-dissolution and the tendency to form poorly soluble compounds on the surface, the area of operation of magnesium protectors is limited to environments with pH = 9.5-10.5. When protecting closed systems, such as tanks, with magnesium protectors, it is necessary to take into account the possibility of the formation of detonating gas due to the release of hydrogen in the cathodic reaction occurring on the surface of the magnesium alloy. The use of magnesium protectors is also associated with the risk of hydrogen embrittlement and corrosion cracking of equipment.
Any metal products are easily destroyed under the influence of certain external factors, most often humidity. To prevent such phenomena, protective corrosion protection is used. Its task is to reduce the potential of the base material and thereby protect it from corrosion.
The essence of the procedure
Protective protection is based on a substance called an inhibitor. This is a metal with increased electronegative qualities. When exposed to air, the protector dissolves. As a result, the base material is preserved even if it is severely affected by corrosion.
Various types of corrosion can be easily defeated if you use cathodic electrochemical methods, which include sacrificial protection. This procedure is an ideal solution when an enterprise does not have the financial capabilities or technological potential to provide complete protection against corrosion processes.
Main advantages
Protective protection of metals from corrosion is good way protection of any metal surfaces. Its use is advisable in several cases:
- When a company does not have enough production capacity to use more energy-intensive techniques.
- When you need to protect small structures.
- If protection of metal products and objects whose surfaces are coated with insulating materials is required.
To achieve maximum efficiency, it is advisable to use tread protection in an electrolytic environment.
When is protection required?
Corrosion occurs on any metal surfaces in a wide variety of areas - from the oil and gas industry to shipbuilding. Protective corrosion protection is widely used in painting tanker hulls. These vessels are constantly exposed to water, and special paint does not always prevent moisture reactions with the metal surface. The use of protectors is a simple and effective solution to the problem, especially if the vessels will be in operation for a long time.

Most metal structures are created from steel, so it is advisable to use protectors that have a negative electrode potential. Three metals are basic for the production of protectors - zinc, magnesium, aluminum. Due to the large potential difference between these metals and steel, the radius of protective action becomes wider, and any types of corrosion are easily eliminated.
What metals are used?

The protective system is built on the basis of various alloys, depending on the specific use of the protectors, for example, the environment in which it will be used. Protective corrosion protection is most often required for iron and steel products, but surfaces made of zinc, aluminum, cadmium or magnesium also require it. A special feature of sacrificial protection is the use of galvanic anodes, which protect pipes from soil corrosion. The calculation of such installations is carried out taking into account a number of parameters:
- current strength in the protector;
- indicators of its resistance;
- degree of protection required for 1 km of pipe;
- number of treads for the same segment;
- the distance that exists between the elements of the protective system.
Pros and cons of different protectors

Protection is built on the basis of protectors building structures from corrosion, pipelines different types(distribution, main, field). However, you need to use them wisely:
- the use of aluminum protectors is advisable in order to protect structures and structures in sea water and the coastal shelf;
- magnesium ones are suitable for use in weakly electrically conductive environments where aluminum and zinc protectors show low efficiency. But they cannot be used if it is necessary to protect the internal surfaces of tankers, tanks, and oil settling tanks, since magnesium protectors are characterized by an increased explosion and fire hazard. Ideally, projectors based on this element should be used for external protection of structures that are used in a fresh environment;
- zinc protectors are completely safe, so they can be used on any objects, even if they have a high level of fire hazard.
If the coating is paintwork

Very often it is necessary to protect an oil or gas pipeline from corrosion, taking into account the paint coating. Combining it with a protector is a passive way to protect structures from corrosion. At the same time, the effectiveness of such an event is not so high, but the following is achieved:
- defects on the coatings of metal structures and pipelines, for example, peeling, cracks, are leveled out;
- the consumption of protective materials is reduced, while the protection itself is more durable;
- the protective current is evenly distributed over the metal surface of the product or object.
Protective corrosion protection in combination with paint and varnish coatings is the ability to distribute protective current precisely to those surfaces that require maximum attention.
About pipeline protection

As you use it metal pipes are exposed to corrosion inside and outside. Plaque appears due to the fact that aggressive substances flow through the pipes, which react with the materials. The internal condition of metal products is affected by high levels of soil moisture. If high-quality protection of building structures from corrosion is not thought out, the following will happen:
- the pipeline will begin to collapse from the inside;
- it will be necessary to carry out preventive inspections of highways more often;
- more frequent repairs will be required, which will result in additional costs;
- it will be necessary to completely or partially stop an oil refining or other industrial complex.
There are several ways to protect pipelines - passive, active. Reducing the aggressiveness of the environment can also serve as a means of protection. To ensure comprehensive protection, the type of pipeline, the method of its installation and interaction with the environment are taken into account.
Passive and active methods of protection
All main methods of protecting pipelines from corrosion come down to performing a number of works. If we talk about passive methods, they are expressed as follows:
- a special installation method, when resistance to corrosion is thought out at the stage of pipeline installation. To do this, an air gap is left between the ground and the pipe, thanks to which nothing will get inside the pipeline. groundwater, no salt, no alkali;
- applying special coatings to pipes that will protect the surface from soil influences;
- treatment with special chemicals, for example, phosphates, which form a protective film on the surface.
Protection scheme based active methods involves the use of electric current and electrochemical ion exchange reactions:

The case for tread protection
As you can see, there are many ways to improve the protective characteristics of pipelines and other metal products. But they all require the expenditure of electrical current. Protective protection against corrosion of pipelines is a more advantageous solution, since all oxide processes are stopped simply by applying alloys of other materials to the surface of metal pipes. The following factors speak in favor of this method:
- cost-effectiveness and simplicity of the process due to the absence of a direct current source and the use of magnesium, zinc or aluminum alloys;
- the possibility of using single or group installations, while the tread protection scheme is thought out taking into account the characteristics of the designed or already constructed facility;
- Possibility of use on any soil and in sea/ocean conditions where it is expensive or impossible to use external current sources.
Tread protection can be used to increase the corrosion resistance of various tanks, ship hulls, and tanks that are used in extreme conditions.
3.1 Cathodic protection
Cathodic protection - the most common type of electrochemical protection. It is used in cases where the metal is not prone to passivation, that is, it has an extended region of active dissolution, a narrow passive region, high values of passivation current (i p) and passivation potential (φ p).
Cathodic polarization can be accomplished by connecting the structure to be protected to the negative pole of an external current source or to a metal having a more electronegative electrode potential. In the latter case, there is no need for an external current source, since a galvanic element is formed with the same direction of current, i.e. the protected part becomes the cathode, and the more electronegative metal, called protector, - anode.
Cathodic protection external current. Cathodic protection using polarization from an external current source is used to protect equipment made of carbon, low- and high-alloy and high-chromium steels, tin, zinc, copper and copper-nickel alloys, aluminum and its alloys, lead, titanium and its alloys. As a rule, these are underground structures (pipelines and cables for various purposes, foundations, drilling equipment), equipment operated in contact with sea water(ship hulls, metal parts of coastal structures, offshore drilling platforms), internal surfaces of apparatus and tanks of the chemical industry. Often cathodic protection is used simultaneously with the application of protective coatings. A decrease in the rate of self-dissolution of a metal during its external polarization is called a protective effect. The main criterion for cathodic protection is the protective potential. The protective potential is the potential at which the rate of metal dissolution takes on the extremely low value acceptable for the given operating conditions. The characteristic of cathodic protection is the value of the protective effect Z, %:Where K 0 [g/(m 2 h)] is the corrosion rate of metal without protection, K 1 [g/(m 2 h)] is the corrosion rate of metal under conditions of electrochemical protection. The protective action coefficient K 3 [g/A] is determined by the formula
K 3 = (m 0 - m i)/i K,
Where m o and m i are the metal mass loss, respectively, without cathodic protection and with its use, g/m 2 ; i to [A/m 2 ] - cathode current density. The cathodic protection diagram is shown in Fig. 51. The negative pole of the external current source 4 is connected to the protected metal structure 1, and the positive pole is connected to the auxiliary electrode 2, which works as an anode. During the protection process, the anode is actively destroyed and is subject to periodic restoration.
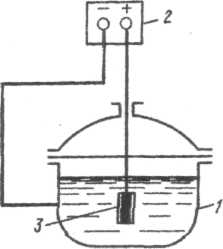
 Cast iron, steel, coal, graphite, scrap metal (old pipes, rails, etc.) are used as anode materials. Since effective resistance to the passage of electric current is provided only by that layer of soil that is located in the immediate vicinity of the anode, it is usually placed in the so-called backfill with a 3-thick layer of coke, to which 3-4 parts (by weight) of gypsum and 1 part are added table salt. The backfill has high electrical conductivity, which reduces the soil-anode contact resistance. The sources of external current for cathodic protection are cathodic protection stations, mandatory elements which are: converter (rectifier), generating current; current supply to the protected structure, reference electrode, anode grounding conductors, anode cable. Cathodic protection stations can be regulated or unregulated. Unregulated cathodic protection stations are used when there are practically no changes in resistance in the current circuit. These stations operate in the mode of maintaining a constant potential or current and are used to protect tanks, storage facilities, high-voltage cables in steel armor, pipelines, etc. Adjustable cathodic protection stations are used when there are stray currents in the system (proximity to electrified transport) , periodic changes in the resistance to current spreading (seasonal fluctuations in temperature and soil humidity), technological fluctuations (changes in the level of the solution and the speed of liquid flow). The adjustable parameter can be current or potential. The frequency of location of cathodic protection stations along the length of the protected object is determined by the electrical conductivity of the operating environment. The higher it is, the greater the distance from each other the cathode stations will be located. To protect structures in water, anodes are installed at the bottom of rivers, lakes, and seas. In this case, backfilling is not required. Cathodic protection of factory equipment (refrigerators, heat exchangers, capacitors, etc.) exposed to aggressive environment, is carried out by connecting an external current source to the negative pole and immersing the anode in this medium (Fig. 52). Cathodic protection by external current is used as an additional means to the insulating coating. In this case, the insulating coating may be damaged. The protective current flows mainly through exposed areas of the metal, which need protection. Cathodic protection with external current is also applied to structures that have significant damage, which makes it possible to stop the further spread of corrosion. The use of cathodic protection is associated with the danger of so-called overprotection. In this case, due to too strong a shift in the potential of the protected structure to the negative side, the rate of hydrogen evolution can sharply increase. The result of this is hydrogen embrittlement or corrosion cracking of materials and destruction of protective coatings. Cathodic protection with external current is impractical in conditions of atmospheric corrosion, in a vaporous environment, in organic solvents, since in this case the corrosive environment does not have sufficient electrical conductivity. Tread protection.
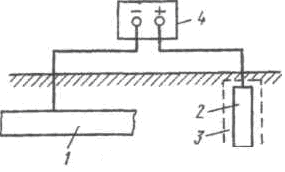
Sacrificial protection is a type of cathodic protection. The pipeline protection scheme is shown in Fig. 53. A more electronegative metal is attached to the protected structure 2 - protector 3, which, dissolving in the environment, protects the main structure from destruction. After complete dissolution of the protector or loss of contact with the protected structure, the protector must be replaced.
Cast iron, steel, coal, graphite, scrap metal (old pipes, rails, etc.) are used as anode materials. Since effective resistance to the passage of electric current is provided only by that layer of soil that is located in the immediate vicinity of the anode, it is usually placed in the so-called backfill with a 3-thick layer of coke, to which 3-4 parts (by weight) of gypsum and 1 part are added table salt. The backfill has high electrical conductivity, which reduces the soil-anode contact resistance. The sources of external current for cathodic protection are cathodic protection stations, mandatory elements which are: converter (rectifier), generating current; current supply to the protected structure, reference electrode, anode grounding conductors, anode cable. Cathodic protection stations can be regulated or unregulated. Unregulated cathodic protection stations are used when there are practically no changes in resistance in the current circuit. These stations operate in the mode of maintaining a constant potential or current and are used to protect tanks, storage facilities, high-voltage cables in steel armor, pipelines, etc. Adjustable cathodic protection stations are used when there are stray currents in the system (proximity to electrified transport) , periodic changes in the resistance to current spreading (seasonal fluctuations in temperature and soil humidity), technological fluctuations (changes in the level of the solution and the speed of liquid flow). The adjustable parameter can be current or potential. The frequency of location of cathodic protection stations along the length of the protected object is determined by the electrical conductivity of the operating environment. The higher it is, the greater the distance from each other the cathode stations will be located. To protect structures in water, anodes are installed at the bottom of rivers, lakes, and seas. In this case, backfilling is not required. Cathodic protection of factory equipment (refrigerators, heat exchangers, capacitors, etc.) exposed to aggressive environment, is carried out by connecting an external current source to the negative pole and immersing the anode in this medium (Fig. 52). Cathodic protection by external current is used as an additional means to the insulating coating. In this case, the insulating coating may be damaged. The protective current flows mainly through exposed areas of the metal, which need protection. Cathodic protection with external current is also applied to structures that have significant damage, which makes it possible to stop the further spread of corrosion. The use of cathodic protection is associated with the danger of so-called overprotection. In this case, due to too strong a shift in the potential of the protected structure to the negative side, the rate of hydrogen evolution can sharply increase. The result of this is hydrogen embrittlement or corrosion cracking of materials and destruction of protective coatings. Cathodic protection with external current is impractical in conditions of atmospheric corrosion, in a vaporous environment, in organic solvents, since in this case the corrosive environment does not have sufficient electrical conductivity. Tread protection.
Sacrificial protection is a type of cathodic protection. The pipeline protection scheme is shown in Fig. 53. A more electronegative metal is attached to the protected structure 2 - protector 3, which, dissolving in the environment, protects the main structure from destruction. After complete dissolution of the protector or loss of contact with the protected structure, the protector must be replaced. ![]()
Figure 53 - Pipeline sacrificial protection scheme The protector works effectively if the transition resistance between it and the environment is low. During operation, a protector, for example zinc, can become covered with a layer of insoluble corrosion products, which isolate it from the environment and sharply increase the contact resistance. To combat this, the protector is placed in a filler 4
- a mixture of salts that creates a certain environment around it, facilitating the dissolution of corrosion products and increasing the efficiency and stability of the tread in the ground 1. The action of the tread is limited to a certain distance. The maximum possible distance of the protector from the protected structure is called the radius of action of the protector. It depends on a number of factors, the most important of which are the electrical conductivity of the medium, the potential difference between the protector and the protected structure, and polarization characteristics. As the electrical conductivity of the medium increases, the protective effect of the protector extends over a greater distance. Thus, the radius of action of a zinc protector when protecting steel in distilled water is 0.1 cm, in sea water 4 m, in a 3% NaCl solution - 6
m. In comparison with cathodic protection by external current, it is advisable to use sacrificial protection in cases where obtaining energy from the outside is associated with difficulties or if the construction of special power lines is not economically profitable. Currently, tread protection is used to combat corrosion of metal structures.
in sea and river water, soil and other neutral
environments Use of tread protection in acidic
environments is limited by the high rate of self-dissolution of the protector. Metals can be used as protectors: Al, Fe, Mg, Zn. However, using pure metals as protectors is not always advisable. For example, pure zinc dissolves unevenly due to its coarse-grained dendritic structure, the surface of pure aluminum is covered with a dense oxide film, magnesium has a high rate of its own corrosion. To give the protectors the required performance properties, alloying elements are introduced into their composition. Cd (0.025-0.15%) and A1 (0.1-0.5%) are introduced into the composition of zinc protectors. They try to maintain the content of impurities such as Fe, Cu, Pb at a level of no more than 0.001-0.005%. Additives are introduced into the composition of aluminum protectors that prevent the formation of oxide layers on their surface - Zn (up to 8%), Mg (up to 5%), as well as Cd, In, Gl, Hg, Tl, Mn, Si (from hundredths to tenths of a percent), contributing to the required change in lattice parameters. Magnesium tread alloys contain Al as alloying additives
(5-7%) and Zn (2-5%); the content of impurities such as Fe, Ni, Cu, Pb, Si is maintained at the level of tenths or hundredths of a percent. Iron as a tread material is used either in its pure form (Fe-armco) or in the form of carbon steels. Zinc protectors are used to protect equipment operating in sea water (sea vessels, pipelines, coastal structures). Their use in slightly salted, fresh water and soils is limited due to the formation of layers of Zn(OH) 2 hydroxide or zinc oxide ZnO on their surface. Aluminum protectors are used to protect structures operating in flowing sea water, as well as to protect port facilities and structures located on the coastal shelf. Magnesium protectors are primarily used to protect small structures in weakly electrically conductive environments where the effectiveness of aluminum and zinc protectors is low - soils, fresh or slightly salty waters. However, due to the high rate of self-dissolution and the tendency to form poorly soluble compounds on the surface, the field of operation of magnesium protectors is limited to environments with pH = 9.5 – 10.5. When protecting closed systems, such as tanks, with magnesium protectors, it is necessary to take into account the possibility of the formation of detonating gas due to the release of hydrogen in the cathode reaction occurring on the surface of the magnesium alloy. The use of magnesium protectors is also associated with the risk of hydrogen embrittlement and corrosion cracking of equipment. As in the case of cathodic protection with external current, the effectiveness of sacrificial protection increases when it is used in combination with protective coatings. Thus, applying a bitumen coating to pipelines significantly improves the distribution of protective current, reduces the number of anodes and increases the length of the pipeline section protected with one protector. If one magnesium anode can protect an uncoated pipeline with a length of only 30 m, then the protection of a bitumen-coated pipeline is effective for a length of up to 8 km.
3.2 Anodic protection
Anodic protection used when operating equipment in highly electrically conductive environments and made of easily passivated materials - carbon, low-alloy stainless steels, titanium, high-alloy iron-based alloys. Anodic protection is promising in the case of equipment made of dissimilar passivating materials, for example, stainless steels of various compositions, welded joints. Anodic protection is carried out by connecting the protected metal structure to the positive pole of an external direct current source or to a metal with a more positive potential (cathode protector). In this case, the potential of the protected metal shifts in the positive direction until a stable passive state is achieved (Fig. 50). As a result, there is not only a significant (thousands of times) reduction in the rate of metal corrosion, but also prevention of the products of its dissolution from entering the manufactured product. Cathodes used for anodic protection from an external current source must have high stability in a corrosive environment. The choice of cathode material is determined by the characteristics of the medium. Materials such as Pt, Ta, Pb, Ni, platinized brass, high-alloy stainless steels, etc. are used. The cathode layout is designed individually for each specific protection case. Materials such as carbon, manganese dioxide, magnetite, and lead dioxide, which have a very positive potential, can be used as a cathode projector. Anodic protection from an external source is based on passing current through the protected object and shifting the corrosion potential towards more positive values. The installation for anodic protection consists of a protection object, a cathode, a reference electrode and an electric current source. The main condition for the possibility of using anodic protection is the presence of an extended region of stable passivity of the metal at a current density of metal dissolution of no more than (1.5-6.0)·10 -1 A/m 2. The main criterion characterizing the state of the metal surface is the electrode potential. Typically, the possibility of using anodic protection for a particular metal or alloy is determined by taking anodic polarization curves. In this case, the following data are obtained: a) the corrosion potential of the metal in the test solution; b) the extent of the area of stable passivity; c) current density in the region of stable passivity. The effectiveness of protection is defined as the ratio of the corrosion rate without protection to the corrosion rate under protection. As a rule, the parameters of anodic protection obtained in laboratory and production conditions are in good agreement with each other. Depending on the specific operating conditions, the area of protective potentials during anodic protection lies 0.3-1.5 V more positive than the free corrosion potential, and the rate of dissolution of metals can decrease thousands of times. A significant limitation in the use of anodic protection is the likelihood of local types of corrosion occurring in the area of the passive state of the metal. To prevent this phenomenon, based on preliminary studies, a value of the protective potential is recommended at which local types of corrosion do not occur or inhibitory additives are introduced into the solution. For example, anodic protection of 12X18N10T steel in chloride solutions in the presence of NO 3 ions prevents the formation of pitting and reduces the rate of steel dissolution by 2000 times. In some cases, due to the increased risk of local corrosion processes, the use of anodic protection is ineffective. A sharp increase The current passivation of metals with increasing temperature of aggressive media limits the use of anodic protection at elevated temperatures. During stationary operation of the installation, the amount of polarization current required to maintain a stable passive state is constantly changing due to changes in the operating parameters of the corrosive environment (temperature, chemical composition, mixing conditions, solution speed, etc.). The potential of a metal structure can be maintained within specified boundaries by means of constant or periodic polarization. In the case of periodic polarization, the current is switched on and off either when a certain potential value is reached or when it deviates by a certain amount. In both cases, the parameters of anodic protection are determined experimentally in laboratory conditions. To successfully use anodic protection, the object must meet the following requirements: a) the material of the apparatus must be passivated in the technological environment; b) the design of the device should not have rivets, the number of cracks and air pockets should be minimal, the welding should be of high quality; c) the cathode and reference electrode in the protected device must be constantly in solution. In the chemical industry, cylindrical devices, as well as heat exchangers, are most suitable for anodic protection. Currently, anodic protection of stainless steels is used for measuring tanks, collectors, tanks, and storage facilities in the production of sulfuric acid, mineral fertilizers, and ammonia solutions. Cases of the use of anodic protection of heat exchange equipment in the production of sulfuric acid and artificial fiber, as well as baths for chemical nickel plating, are described. The anodic protection method has relatively limited application, since passivation is effective mainly in oxidizing environments in the absence of active depassivating ions, such as chlorine ions for iron and stainless steels. In addition, anodic protection is potentially dangerous: in the event of a power interruption, the metal may be activated and undergo intense anodic dissolution. Therefore, anodic protection requires a careful control system. Unlike cathodic protection, the corrosion rate with anodic protection never decreases to zero, although it may be very small. But the protective current density here is much lower, and electricity consumption is low. Another advantage of anodic protection is its high dissipative ability, i.e. the possibility of protection at a distance further from the cathode and in electrically shielded areas.3.3 Oxygen protection
Oxygen protection is a type of electrochemical protection in which the potential of the protected metal structure is shifted in a positive direction by saturating the corrosive environment with oxygen. As a result of this, the speed of the cathodic process increases so much that it becomes possible to transfer steel from an active to a passive state.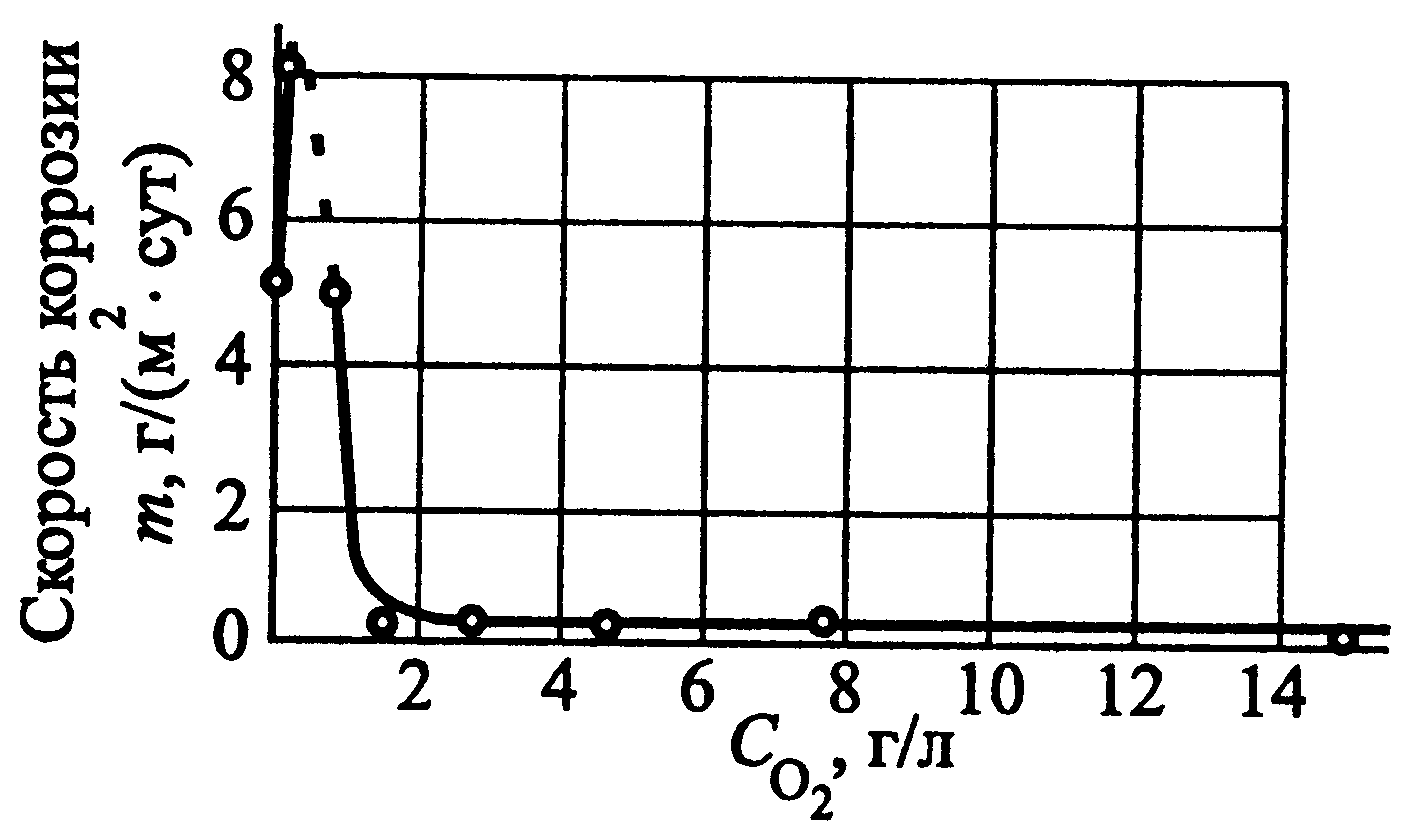
Figure 54 - Dependence of the corrosion rate of low-alloy steel in water at a temperature of 300 °C on the oxygen concentration in water Since the value of the critical passivation current of Fe-Cr alloys, which includes steels, significantly depends on the chromium content in them, its efficiency increases with increasing chromium concentration in the alloy. Oxygen protection is used for corrosion of thermal power equipment operating in water at high parameters (high temperature and pressure). In Fig. 54 The dependence of the corrosion rate of low-alloy steel on the oxygen concentration in high-temperature water is presented. As can be seen, an increase in the concentration of oxygen dissolved in water leads to an initial increase in the corrosion rate, a subsequent decrease and further stationarity. Low steady-state dissolution rates of steel (10-30 times lower than those without protection) are achieved at an oxygen content in water of ~ 1.8 g/l. Oxygen protection of metals has found application in nuclear energy.
4. Development and production of new
construction materials increased corrosion resistance
Improving the anti-corrosion properties of the metal materials themselves is carried out:
- elimination of impurities from metals and alloys that accelerate corrosion processes; doping.
5 Transition in a number of structures from metal
to chemically resistant non-metallic materials
Non-metallic materials are an additional reserve for organizing anti-corrosion protection. Individual devices or parts for them can be made from glass, ceramics, glass ceramics, vinyl plastic, faolite, graphite and other non-metallic materials. Their distinctive feature is high corrosion resistance in many aggressive environments. Non-metallic materials are discussed in detail in Chapter V, Part 4.
6 Rational design and operation of metal structures and parts
When designing chemical production, as a rule, the main attention is paid to analyzing the nature of the aggressive environment and the conditions of the process. Based on these data, a material with sufficient chemical resistance is selected. However, the structural material that is most resistant in a given corrosive environment does not in all cases prevent the danger of rapid corrosion destruction. Therefore, the rational design of individual components and devices deserves just as close attention. In many cases, poor design can cause the formation of stagnant zones, gaps, stress concentrations and other phenomena that contribute to the occurrence and progression of corrosion. At the same time, already at the design stage it is possible to provide such Constructive decisions individual components of the device, which will significantly reduce or eliminate the possibility of the corrosion process. When designing equipment, you should pay attention to the nature of the metal surface treatment, the contact of connecting elements made of different materials, the mode of distribution of coolant flows, the presence of cracks and gaps, and the possibility of the formation of stagnant zones. A smooth metal surface has fewer defects in the form of scratches, irregularities, etc. Dirt, dust and other substances accumulate more easily on a rough surface. This is especially typical for the production of fertilizers and salts. In this case, metal equipment and structures with a rough surface are covered with various substances. If these substances are hygroscopic, then they stick to the metal surface, creating local pockets with a high concentration of electrolyte, which contributes to increased corrosion. If the device being designed contains parts made of various metal materials, then there is a danger of contact corrosion. In this case, even at the design stage, measures must be taken to prevent or mitigate this phenomenon. Usually one of two possible techniques is used. Contacting parts from dissimilar metals are made with different surface sizes. In this case, the part with the smallest surface should be made of a more noble metal (valve bushings, piston rings pumps, etc.). If this method turns out to be impossible, then parts made of different materials are isolated from each other. Particular attention should be paid to the properties of the cushioning material. It must be inert with respect to the working environment and have high wear resistance. Some insulating materials (felt, asbestos, wood) can absorb and retain moisture and thus be a source of increased corrosion. Some polymer materials, subject to aging over time, when in contact with water, can release corrosive agents that accelerate the destruction of metals. Therefore, insulating materials are often impregnated with tar or bitumen, and the polymer materials used are subjected to special studies to determine the risk of releasing aggressive agents. Many processes occur when elevated temperatures. The metal surface in contact with coolants is subject to additional corrosive effects. The higher the temperature, the more intense the destruction of the metal. For example, heat exchangers are one of the least resistant types of equipment. In 92% of cases, the cause of heat exchanger failure is corrosion of heat-transfer surfaces. When designing devices, it is necessary to provide for a uniform distribution of heat flow and eliminate the possibility of local overheating. In many industrial devices in which high-temperature processes occur, such measures are provided. For example, in the production of ammonia, a channel is provided between the catalyst box, in which the reaction takes place at a temperature of 350-420ºC, and the body of the apparatus, inside which cold gas circulates. This design technique protects the walls of the apparatus from overheating. If jacketed reactors are used in a chemical process, a stirrer must be installed inside the reactor to ensure uniform movement of the liquid past the heat transfer surface. Coils, boilers and other equipment for heating process media must be immersed in liquid. For heat exchange equipment, the most common types of corrosion are local ones, such as pitting, crevice, and intergranular. At structural design Welding locations and methods must be designated. When welding metals, large tensile stresses are created in the weld zone and near-heat zone. In zones located along the seam, where the metal is heated above critical temperatures, the structure of the metal changes. This may cause the metal to crack. When designing welded assemblies and parts, a number of measures should be taken: avoid accumulation of seams, exclude spot welding, in which the stress concentration is especially high, use local annealing, etc. Moisture accumulation in various structural elements contributes to the development of corrosion. Therefore, when creating various structures, provisions are made for the possibility of ventilating cavities, the presence of drainage holes, etc. Gaps and cracks are very dangerous zones in the equipment in terms of corrosion. Concentration of the working solution can occur in them, aeration is disrupted, which will inevitably lead to the development of local corrosion. From this point of view, intermittent welds are dangerous, in which, due to the loose fit of the material to each other, cracks and gaps are formed, which are the cause of crevice corrosion. The formation of stagnant zones of liquid in apparatus and pipelines greatly increases the possibility of corrosion due to the formation of microvapor of uneven aeration. This is also facilitated by the deposition of various sediments in the stagnant zone. The product should not have various recesses, grooves and grooves in which moisture can accumulate. Structural elements should be as streamlined as possible, this facilitates the evaporation of moisture. In rationally designed units, the possibility of accumulation of moisture and corrosion products is eliminated, and the possibility of removing sediment is provided. There are other design requirements that ensure the creation of products that are least susceptible to corrosion. These include: requirements for the general layout and arrangement of elements, taking into account the possibility of applying and renewing various coatings during operation and during repairs, taking into account the operating features of products, etc.
BIBLIOGRAPHY
Anodic protection. Use of passivity in corrosion protection practice.
Many metals are in a passive state in some aggressive environments. Chromium, nickel, titanium, zirconium easily pass into a passive state and maintain it stably. Often, alloying a metal that is less prone to passivation with a metal that passivates more easily leads to the formation of fairly well passivated alloys. An example is the varieties of Fe-Cr alloys, which are various stainless and acid-resistant steels, resistant, for example, in fresh water, atmosphere, nitric acid, etc. This use of passivity in corrosion protection technology has been known for a long time and is of great practical importance. But recently, a new direction has emerged in the protection of metals in such oxidizers that by themselves are not capable of causing passivity. It is known that a shift in the potential of the active metal in the negative direction should reduce the corrosion rate. If the potential becomes more negative than the equilibrium one in a given environment, then the corrosion rate should become zero (cathodic protection, use of protectors). Obviously, in a similar way, but due to anodic polarization from an external source of electrical energy, it is possible to transfer a metal capable of this into a passive state and thereby reduce the corrosion rate by several orders of magnitude. The consumption of electrical energy should not be large, since the current strength is generally very small. There are requirements that a system must meet in order for anodic protection to be applied to it. First of all, you need to reliably know the anodic polarization curve for the selected metal in a given aggressive environment. The higher i P, the greater the current required to transfer the metal to a passive state; the smaller i nn , the less energy consumption is required to maintain passivity; the wider the range Δφ n, the larger potential fluctuations can be tolerated, i.e. the easier it is to maintain the metal in a passive state. You need to be sure that in the region of Δφ n the metal corrodes evenly. Otherwise, even with a small value of i nn the formation of ulcers and through corrosion of the product wall is possible. The shape of the protected surface can be quite complex, which makes it difficult to maintain the same potential value over the entire surface; in this regard, a large value of Δφ n is especially desirable. Of course, sufficiently good electrical conductivity of the medium is also required. The use of anodic protection is advisable in highly aggressive environments, for example in the chemical industry. If there is a liquid-gas interface, it must be borne in mind that anodic protection cannot extend to the metal surface in a gaseous environment, which, however, is also typical for cathodic protection. If the gas phase is also aggressive or there is a restless interface, which leads to liquid splashing and settling of droplets on the metal above the interface, if periodic wetting of the product wall occurs in a certain zone, then the question of other methods of protecting the surface above a constant liquid level has to be raised. Anodic protection can be carried out in several ways. 1. Simple application of a constant emf. from an external source of electrical energy. The positive pole is connected to the protected product, and relatively small cathodes are placed near its surface. They are placed in such quantity and at such a distance from the protected surface to ensure as uniform anodic polarization of the product as possible. This method is used if Δφ n is large enough and there is no danger, with some inevitable uneven distribution of the anode potential, activation or repassivation, i.e. going beyond the limits of Δφ n. In this way, products made of titanium or zirconium can be protected in sulfuric acid. You just need to remember that for passivation, you will first need to pass a higher current, which is associated with the transfer of the potential beyond φ n . For the initial period, it is advisable to have an additional source of energy. One should also take into account the greater polarization of cathodes, the current density of which is high due to their small size. However, if the region of the passive state is large, then a change in the cathode potential even by a few tenths of a volt does not pose a danger. Periodic switching on and off of the protection current when the product is already passivated. When the anode current is turned on, the potential of the product shifts to the negative side, and depassivation may occur. But since sometimes this happens quite slowly, simple automation can ensure that the protective current is turned on and off in right time. When the potential reaches the value φ nn ", i.e. before the start of repassivation, the current is turned off; when the potential moves negative to φ nn (start of activation), the current is switched on again. The potential shift to the cathode side occurs the slower, the smaller φ nn . The closer the potential was to the value φ nn ", the slower it shifts to the negative side (in the direction of φ nn) when the current is turned off. For example, for chromium in a 0.1 N solution of H 2 SO 4 at 75 ° C, if the current is turned off at φ =0.35V, activation will occur in 2 hours; switching off the current at φ =0.6 V causes activation via 5 h; switching off at φ = 1.05 V increases the start time of activation to more than 127 hours. So big time , necessary for depassivation, allows significant interruptions in the current supply. Then the same installation can serve several objects. The dependence of the passivation time on the switching potential is easily explained using the concept of phase oxide (a thicker oxide layer is formed, the dissolution of which takes longer). It is more difficult to explain this phenomenon by desorption of passivating oxygen. Of course, with increasing positive potential, the bond strength in the adsorption layer should increase. But when the current is turned on, the discharge of the double layer occurs relatively quickly, although the adsorption layer may persist for a long time. 3. If the region of the passive state (Δφ nn) is small, then it is necessary to use a potentiostat that maintains a given potential value (relative to a certain reference electrode) within narrow limits. The potentiostat must be capable of delivering high current. Currently, there are already a number of installations for anodic protection implemented on an industrial scale. Products made from ordinary carbon steel are also protected. With anodic protection, the service life of the equipment not only increases, but also the contamination of the aggressive environment with corrosion products is reduced. For example, in oleum, carbon steel corrodes very slowly and in this sense does not need protection. But in the vessels for storing this product, it becomes contaminated with iron. Thus, without anodic protection in one of the industrial installations, the iron content in oleum was ~ 0.12%. After applying the protection, the iron concentration decreased to ~ 0.004%, which corresponds to its content in the original product. Contamination of chemical industry products with impurities of metal compounds, which is a consequence of equipment corrosion, is in many cases very undesirable and even unacceptable. However, the use of anodic protection is associated with significant difficulties. While cathodic protection can be used to protect many metals immersed in any electrically conductive medium, such as solid or liquid, anodic protection is used only to protect entire sections of chemical plants that are made of metal that can be passivated in the working environment. This is precisely what limits its use. In addition, anodic protection is potentially dangerous, since if the current supply is interrupted without immediate restoration of protection, very rapid dissolution will begin in the area in question, since a break in the film forms a low-resistance path under conditions of anodic polarization of the metal. The use of anodic protection requires careful design of the chemical plant. The latter must have a monitoring system such that any loss of protection immediately attracts the operator's attention. For this purpose, only a local increase in the anode current may be sufficient, but in worst case The entire installation may need to be emptied immediately. Anodic protection does not provide resistance in the presence of aggressive ions. Thus, chloride ions destroy the passive film, and therefore their concentration must be kept low, with the exception of the protection of titanium, which can be passivated in hydrochloric acid. Under conditions of anodic protection, the electrolytes have a good dissipative ability and therefore a relatively small number of electrodes is required to maintain its established protection. However, when designing anodic protection installations, it should be taken into account that in conditions preceding passivation, the dissipation ability is worse. Anodic protection consumes very little energy and can be used to protect common structural metals that can be passivated, such as carbon and stainless steel, in many environments. This protection is easily controlled and measured and does not require expensive metal surface treatment, since it uses the spontaneous reaction effect between the walls of the containers and their contents. The method is elegant, and its use is likely to expand once the difficulties of measurement and control are overcome.Coatings as a method of protecting metals from corrosion.
Protection of metals, based on changes in their properties, is carried out either by special treatment of their surface or by alloying. Treatment of the metal surface in order to reduce corrosion is carried out in one of the following ways: covering the metal with surface passivating films from its poorly soluble compounds (oxides, phosphates, sulfates, tungstates or combinations thereof), creating protective layers from lubricants, bitumen, paints, enamels, etc. P. and by applying coatings of other metals that are more resistant in these specific conditions than the metal being protected (tinning, galvanizing, copper plating, nickel plating, chrome plating, lead, rhodium plating, etc.). The protective effect of most surface films can be attributed to the mechanical isolation of the metal from the environment they cause. According to the theory of local elements, their effect should be considered as a result of an increase electrical resistance(Fig. 8). An increase in the stability of iron and steel products when their surface is coated with deposits of other metals is due to both the mechanical insulation of the surface and a change in its electrochemical properties. In this case, either a shift in the reversible potential of the anodic reaction towards more positive values (plating with copper, nickel, rhodium), or an increase in the polarization of the cathodic reaction - an increase in the hydrogen overvoltage (zinc, tin, lead) can be observed. As the diagrams show, all these changes reduce the corrosion rate. Metal surface treatment is used to protect machinery, equipment, apparatus and household items for temporary protection during transportation, storage and conservation (lubricants, passivating films) and for longer-term protection during their operation (varnishes, paints, enamels, metal coatings). A common disadvantage of these metals is that when the surface layer is removed (for example, due to wear or damage), the corrosion rate at the damaged area increases sharply, and re-application of the protective coating is not always possible. In this regard, alloying is a much more effective (albeit more expensive) method of increasing the corrosion resistance of metals. An example of increasing the corrosion resistance of a metal by alloying is alloys of copper and gold. To reliably protect copper, it is necessary to add a significant amount of gold to it (at least 52.2 at.%). Gold atoms mechanically protect copper atoms from their interaction with the environment. An incomparably smaller amount of alloying components is required to increase the stability of the metal if these components are capable of forming protective passivating films with oxygen. Thus, the introduction of chromium in an amount of several percent sharply increases corrosion resistanceInhibitors.
The corrosion rate can also be reduced by changing the properties of the corrosive medium. This is achieved either by appropriate treatment of the environment, as a result of which its aggressiveness is reduced, or by introducing small additives of special substances, so-called corrosion retarders or inhibitors, into the corrosive environment. Treatment of the environment includes all methods that reduce the concentration of its components, especially those that are corrosive. For example, in neutral salt environments and fresh water, one of the most aggressive components is oxygen. It is removed by deaeration (boiling, distillation, bubbling of inert gas) or lubricated with appropriate reagents (sulfites, hydrazine, etc.). A decrease in oxygen concentration should almost linearly reduce the limiting current of its reduction, and, consequently, the rate of metal corrosion. The aggressiveness of the medium also decreases when it is alkalized, the total salt content is reduced and more aggressive ions are replaced with less aggressive ones. When anti-corrosion treatment of water to reduce scale formation, its purification with ion-exchange resins is widely used. Corrosion inhibitors are divided, depending on the conditions of their use, into liquid-phase and vapor-phase or volatile. Liquid-phase inhibitors are in turn divided into corrosion inhibitors in neutral, alkaline and acidic environments. Anionic inorganic substances are most often used as inhibitors for neutral solutions. Their inhibitory effect is apparently associated either with the oxidation of the metal surface (nitrites, chromates), or with the formation of a film of a sparingly soluble compound between the metal, this anion and, possibly, oxygen (phosphates, hydrophosphates). The exception in this regard is the salts of benzoic acid, the inhibitory effect of which is associated mainly with adsorption phenomena. All inhibitors for neutral media inhibit predominantly the anodic reaction, shifting the stationary potential in a positive direction. To date, it has not yet been possible to find effective inhibitors of metal corrosion in alkaline solutions. Only high molecular weight compounds have some inhibitory effect. Almost exclusively organic substances containing nitrogen, sulfur or oxygen in the form of amino, imino, thio groups, as well as carboxyl, carbonyl and some other groups are used as acid corrosion inhibitors. According to the most common opinion, the effect of acid corrosion inhibitors is associated with their adsorption at the metal-acid interface. As a result of the adsorption of inhibitors, inhibition of the cathodic and anodic processes is observed, reducing the corrosion rate. The effect of most acid corrosion inhibitors is enhanced by the simultaneous introduction of additives of surface-active anions: halides, sulfides and thiocyanates. Vapor phase inhibitors are used to protect machines, apparatus and other metal products during their operation in an air atmosphere, during transportation and storage. Vapor phase inhibitors are introduced into conveyors, into packaging materials, or placed in close proximity to the operating unit. Due to the sufficiently high vapor pressure, volatile inhibitors reach the metal-air interface and dissolve in the moisture film covering the metal. They are then adsorbed from the solution on the metal surface. The inhibitory effects in this case are similar to those observed with the use of liquid-phosphate inhibitors. As vapor-phase inhibitors, amines with low molecular weight are usually used, into which appropriate groups are introduced, for example NO 2 or CO 2. Due to the peculiarities of the use of vapor-phase inhibitors, increased requirements are placed on them regarding their toxicity. Inhibition is a complex defense and its successful application in a variety of settings requires extensive knowledge.Protective protection and electrical protection.
Protective protection is used in cases where a structure (underground pipeline, ship hull) located in an electrolyte environment (sea water, underground, soil water, etc.) is protected. The essence of such protection is that the structure is connected to the tread - more active metal than the metal of the protected structure. Magnesium, aluminum, zinc and their alloys are usually used as protectors when protecting steel products. During the corrosion process, the protector serves as an anode and is destroyed, thereby protecting the structure from destruction. As the protectors deteriorate, they are replaced with new ones. Electrical protection is also based on this principle. The structure, located in the electrolyte environment, is also connected to another metal (usually a piece of iron, a rail, etc.), but through an external current source. In this case, the protected structure is connected to the cathode, and the metal is connected to the anode of the current source. Electrons are taken away from the anode by a current source, the anode (protecting metal) is destroyed, and the oxidizing agent is reduced at the cathode. Electrical protection has an advantage over tread protection! the radius of action of the first is about 2000 m, the second is about 50 m. Changes in the composition of the environment. To slow down the corrosion of metal products, substances (most often organic) called corrosion inhibitors or inhibitors. They are used in cases where the metal must be protected from corrosion by acids. Soviet scientists have created a number of inhibitors (preparations of the brands ChM, PB, etc.), which, when added to acid, slow down the dissolution (corrosion) of metals hundreds of times. IN last years Volatile (or atmospheric) inhibitors have been developed. They impregnate paper that is used to wrap metal products. Inhibitor vapors are adsorbed on the metal surface and form a protective film on it. Inhibitors are widely used in chemical descaling of steam boilers, removal of scale from processed products, as well as during storage and transportation of hydrochloric acid in steel containers. Inorganic inhibitors include nitrites, chromates, phosphates, and silicates. The mechanism of action of inhibitors is the subject of research by many chemists.Creation of alloys with anti-corrosion properties.
By introducing up to 12% chromium into the steel composition one obtains stainless steel resistant to corrosion. Additions of nickel, cobalt and copper enhance the anti-corrosion properties of steel, as the susceptibility of alloys to passivation increases. The creation of alloys with anti-corrosion properties is one of the important areas in the fight against corrosion losses.Goals, objectives and research methods
Purpose given research work is the study of corrosion and restoration of architectural values of the city of Tsivilsk and the Ivanovo rural administration. Based on the goal, the following were set: tasks:Analyze the literature on this issue.
Study methods of protecting metal products from corrosion.
Conduct a study to identify the architectural values of the city of Tsivilsk and the Ivanovo rural administration.
Suggest ways to protect the objects under study.
- Collection and analysis of theoretical information. Search for cultural monuments: monuments, memorial plaques, etc. Observations in order to determine the material from which the architectural value is made and possible processes of destruction.
Research results
Research into the architectural values of the city of Tsivilsk and the Ivanovo rural administration was carried out from November to December 2005. During the tour of Tsivilsk, the following attractions were identified:- Monument dedicated to the 400th anniversary of the city of Tsivilsk. Monument to fallen soldiers in Velikaya Patriotic War. Monument to V.I. Lenin. Exposition in front of the District Military Commissariat. Monument in honor of the WWII participant, resident of Tsivilsk A. Rogozhkin. Monument in honor of the WWII participant, resident of Tsivilsk Silantiev. Exposition in front of kindergarten No. 4.
| Architectural value | Appearance(material, shape) | Methods of protection against corrosion |
||
| carried out | the most optimal |
|||
| Tsivilsk | Monument dedicated to the 400th anniversary of Tsivilsk | |||
| Monument to V.I. Lenin | A marble Lenin with an outstretched arm, covered with silver paint, is installed on a concrete stand about 1 meter high. The total height of the composition is about 2.5-3 meters. | Regular painting of the monument, including the pedestal. However, this does not protect against mechanical damage under the influence of wind, water and sun. There is a noticeable crack on the leg. | Restoration work is required to eliminate the crack. It is advisable to use special alkyd paints for applying to the surface of the monument. | |
| Its architecture and material are similar to the Lenin monument. The composition includes a soldier made of marble, covered with silver paint, located on a concrete stand 1 meter high. The stand is lined with metal sheets. The total height is about 5 meters. Nearby is a memorial plaque, which is a long brick wall on which galvanized sheets are mounted with the names of WWII participants who did not return from the front. | Painting is carried out, however, due to the high height of the monument, it is not done regularly. Does not corrode. | It is necessary to clean the monument from dried leaves and branches. | ||
| Exposition in front of the District Military Commissariat | A cannon mounted on a brick stand. Height is about 2 meters. Metal (steel), green. There is a 4 cm deep notch on the gun barrel. | The gun is regularly painted by commissariat workers with green alkyd paint, although in a slightly different shade than the original color of the product. A notch on the trunk contributes to destruction. | Tread protection is possible; rivets and zinc plates can be used as a protector. |
|
| Monument in honor of the WWII participant, resident of Tsivilsk A. Rogozhkin | There is a green marble slab on a concrete stand. A bas-relief made of a corrosion-resistant alloy with the image of the sailor Silantiev is mounted into the slab. | Restoration of the monument has not been carried out for a very long time. Cracks are visible on the marble slab. The bas-relief does not corrode, but chipped parts are noticeable. | Care and timely replacement of marble slabs, which are most susceptible to destruction. |
|
| Monument in honor of the WWII participant, resident of Tsivilsk Silantiev | Similar to the monument in honor of Rogozhkin. A bas-relief made of durable alloy with the image of Silantyev is mounted on a marble stand in the form of a triangle. | The bas-relief is not subject to corrosion. | Timely coating of load-bearing structures with protective compounds. | |
| Exposition in front of kindergarten No. 4. | Statues of two pioneers with bugles. | |||
| n. Experienced | Monument to fallen soldiers in the Great Patriotic War | On the white brick wall there is a bas-relief depicting warring soldiers, painted with golden paint. | Does not corrode. Painted regularly. Cracks are noticeable on the bas-relief. | Repairing the crack. |
| With. Ivanovo | Memorial plaque to fallen soldiers in the Great Patriotic War | |||
| Sinya-Kotyaki village | Monument in honor of the 60th anniversary of Victory in the Great Patriotic War (erected in July 2004). | The monument is made of marble chips, lined with white brick. The inscriptions on the memorial are painted golden. | It is practically not subject to corrosion. Brick can be destroyed by wind, sun and water. | More regular painting of letters, timely replacement of supporting structures. |

conclusions
As a result of the study of the architectural values of the city of Tsivilsk and the Ivanovo rural administration, we received important information about the condition of the monuments and methods of their preservation.Spontaneous oxidation of metals, harmful to industrial practice(reducing the durability of products) is called corrosion. The environment in which metal corrodes is called corrosive, or aggressive.
There are many ways to protect metals from corrosion. The most effective among them are protection, inhibition, creation of a protective layer (varnishes, paints, enamels) and anti-corrosion alloys.
Six main attractions have been identified in the city of Tsivilsk. Each studied settlement of the Ivanovo rural administration contains one architectural value dedicated to the Great Patriotic War. In general, these monuments are complex compositions made of marble with the addition of metal fragments. Only the cannon in front of the Regional Military Commissariat is exposed to corrosion.
To protect the objects under study from corrosion, timely care and cleaning is recommended; for some (the Lenin monument, the monument in honor of fallen soldiers in Tsivilsk) regular painting with special compounds is recommended. The monument in honor of the sailor Rogozhkin requires restoration of the supporting structure. For the gun that is most susceptible to corrosion, we also offer tread protection.
List of used literature
- Akhmetov N.S., General and inorganic chemistry. - M.: graduate School, 1989 Nekrasov B.V., Textbook of general chemistry. - M.: Chemistry, 1981 Cotton F., Wilkinson J., Fundamentals of inorganic chemistry. - M.: Mir, 1979 Karapetyants M.Kh., Drakin S.I., General and inorganic chemistry. - M.: Chemistry, 1993 Yakovlev A. A. In the world of stone. M.: Detgiz, 1991
1 From the Latin corrode - to corrode.




